Recently, Prof. Wang Jianjun & Liu Hong's team from the State Key Laboratory of Crystal Materials of Shandong University made a series of new progress in water splitting hydrogen production. Relevant results have been published in Advanced Science, Applied Catalysis B: Environmental, Small, and some of the results were selected as cover articles.
Hydrogen is the most promising clean energy in the 21st century owing to its advantages of high energy density and no pollution, which can be used in various forms such as combustion and fuel cell. Splitting water hydrogen production using renewable energy such as solar and wind energy can not only obtain high purity hydrogen to replace traditional fossil fuels but also overcome the shortcomings of the above renewable energy such as low energy density and intermittence, thereby effectively realizing the storage and utilization of renewable energy. Based on this, the development of high-efficiency water splitting catalytic electrode materials is particularly important.
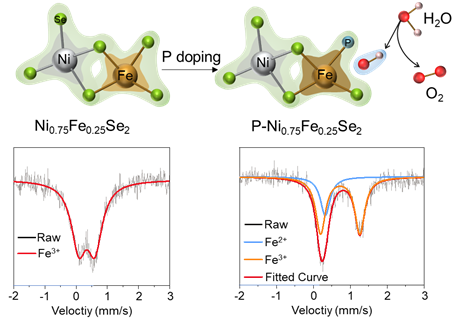
Schematic diagram of the electronic structure changes for P-Ni0.75Fe0.25Se2.
Studying the relationship between the electronic structure of the active site and the catalytic activity is helpful to understand the mechanism of the active site and reveal the corresponding catalytic mechanism, so as to achieve the goal of directional regulation of the catalytic activity of the catalyst. Although great progress has been made in the regulation of the electronic structure of single-metal-based catalysts, less attention has been paid to the precise regulation of the electronic structure of bimetal-based and multi-metal-based catalysts. Therefore, developing a precise regulation method of the electronic structure of bimetallic catalysts is of great significance to improve the electrocatalytic activity and identify the active center.
Researchers selected Ni0.75Fe0.25Se2 with a clear structure and unoptimized performance as the model system to study the precise regulation of electronic structure. Besides, the effect of P doping on the electronic structure for metal active sites in Ni0.75Fe0.25Se2 was studied in detail by means of theoretical calculation, XANES spectra, Mössbauer spectra, and X-ray photoelectron spectroscopy. Related mechanism of the electrocatalytic reaction process was revealed and the preparation of efficient and stable electrocatalyst was realized. The work entitled ‘Highly Efficient Oxygen Evolution Reaction Enabled by Phosphorus Doping of the Fe Electronic Structure in Iron–Nickel Selenide Nanosheets’ was published online in Advanced Science (Adv. Sci., 2021, 2101775). PhD student Huang Yuan from the School of Crystal Materials is the first author of this paper.
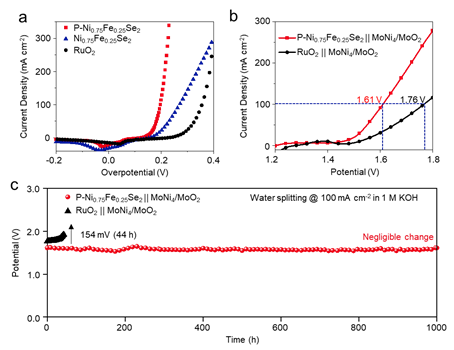
Electrocatalytic Activity of P-Ni0.75Fe0.25Se2.
In order to realize the industrial amplification of P-Ni0.75Fe0.25Se2 electrocatalyst, the researchers conducted an in-depth study on the activity and stability of the designed material. The results show that the optimal P-Ni0.75Fe0.25Se2 exhibits an extremely low overpotential of 156 mV at 10 mA cm−2and the highly efficient activity remains stable for up to 1000 h at 300 mA cm−2. More importantly, the water-alkali electrolyzer of P-Ni0.75Fe0.25Se2|| MoNi4/MoO2 requires only 1.45 V to reach current densities of 10 mA cm−2. The work entitled ‘Precisely Engineering the Electronic Structure of Active Sites Boosts the Activity of Iron-Nickel Selenide on Nickel Foam for Highly Efficient and Stable Overall Water Splitting’ was published online in Applied Catalysis B: Environmenta (Appl. Catal., B, 2021, 120678.). PhD student Huang Yuan from the School of Crystal Materials is the first author of this paper.
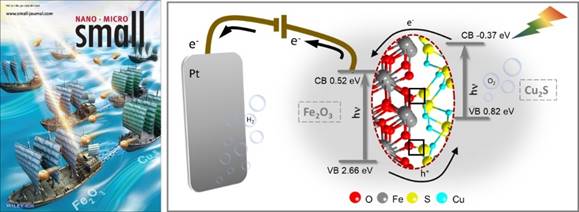
In addition, hematite (α-Fe2O3) is one of the most investigated photoanode materials with suitable band gap, stable properties and abundant reserves. However,the photoelectrochemical performance of hematite is severely restricted by serious charge recombination and slow oxygen evolution reaction kinetics. The effective separation of photogenerated carriers can be realized by constructing hetero junction and forming built-in electric field. However, there are usually two solid phase interfacial resistance and carrier recombination centers, which will limit the effective charge transfer and affect the photoelectrochemical performance. The formation of chemical bond at the heterogeneous interface can consolidate the synergistic effect by reducing interfacial contact resistance, optimizing bulk electronic structure, reconstructing active sites, and adjusting the adsorption/desorption process for reaction intermediates. We cooperated with Professor Artur Braun of Swiss Federal Institute of Materials Science and Technology to construct Cu2S/Fe2O3 heterojunction, and found that the formation of covalent S-O bond between Cu2S and Fe2O3 can significantly improve the photoelectrochemical performance and stability. Compared with bare Fe2O3, the Cu2S/Fe2O3 heterojunction enhances the charge separation and transfer, enlarges the light absorption range, and reduces the charge recombination rate. In addition, due to the photothermal properties of Cu2S, the heterostructure exhibits a higher local temperature under light, which is conducive to improving the rate of oxygen evolution reaction. Therefore, the photocurrent density of the heterostructure increases by 177% at 1.23V vs. RHE. Related work was published in the authoritative journal Small with the title "Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting", and was selected as the Inside Front Cover of the journal. The first author is Zhang Yan, a postgraduate student from the School of Crystal Materials, Shandong University. Related results were also extended to the BiVO4 system, which achieved significantly enhanced water splitting performance (J. Phys. Chem. C, 2021, 125, 15890, Supplementary Cover). The first author is Zhu Shishi, a postgraduate student from the School of Crystal Materials, Shandong University.
The related research work has been supported and funded by the National Natural Science Foundation of China, Shandong Natural Science Foundation, Shenzhen Science and Technology Innovation Commission, State Key Laboratory of Crystal Materials of Shandong University, Qilu Young Scholars Program, etc.
