Recently, Prof. Li Shengying’ s laboratory from the State Key Laboratory of Microbial Technology (Microbial Technology Institute) in collaboration with Prof. Wang Binju’ s group from Xiamen University reported a new mechanism for carbon-carbon bond scission by cytochrome P450 peroxygenases through carefully analyzing the activities of three representative CYP152 peroxygenases (OleTJE, P450SPα, P450BSβ) towards α,β-unsaturated fatty acids as substrate probes. In this unusual mechanism, the adjacent water molecule derived from H2O2 activation is responsible for the C-C bond cleavage. The related research article entitled “Unexpected Reactions ofα,β-Unsaturated Fatty Acids Provide Insight into the Mechanisms of CYP152 Peroxygenases” was published on Angewandte Chemie International Edition.
Cytochrome P450 enzymes catalyze diverse oxidative reactions towards a myriad of natural and unnatural substrates, thus being named the most versatile biocatalysts in nature. A vast majority of P450s share a common monooxygenation mechanism that requires O2, NAD(P)H, and redox partner(s) as the oxygen donor, electron source, and electron shuttle(s), respectively. However, a handful of P450s such as CYP152 peroxygenases have evolved the ability to directly utilize H2O2 as the sole oxygen and electron donor to drive the P450 catalysis via a so-called peroxide shunt pathway.
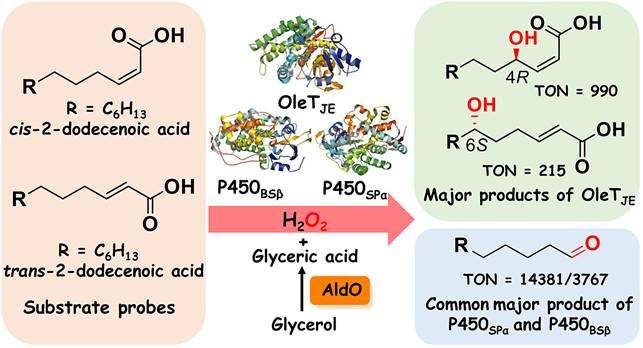
The P450 peroxygenase OleTJE (CYP152L1) from Jeotgalicoccus sp. ATCC 8456 has been attracting great attention from the fields of both biofuels and biomaterials in the past decade because it catalyzes a single-step oxidative decarboxylation of fatty acids to form α-olefins as value-added products using H2O2 as cofactor. By contrast, the fatty acid α-hydroxylase P450SPα (CYP152B1) from Sphingomonas paucimobilis and the fatty acid β-hydroxylase P450BSβ (CYP152A1) from Bacillus subtilis preferentially convert fatty acids into α- and β-hydroxy fatty acids, respectively. The molecular basis of the unique chemo- and regioselectivity of P450 peroxygenases of CYP152 family, especially how the conformational and electronic properties of substrate affect the catalytic outcome of these enzymes, is one of the frontiers and hot areas in the field of enzymology.
In this work,cis- and trans-2-dodecenoic acid (α,β-unsaturated C12fatty acids) were designed and used as substrate probes for comparative catalytic analysis (chemoselectivity, product profile and catalytic efficiency) of OleTJE, P450BSβ and P450SPα with complementary experimental and computational approaches. The unexpected 6S-hydroxylation of thetrans-isomer and 4R-hydroxylation of thecis-isomer by OleTJE, and molecular docking results suggest that the unprecedented selectivity is likely due to OleTJE’s preference of C2-C3cis-configuration.
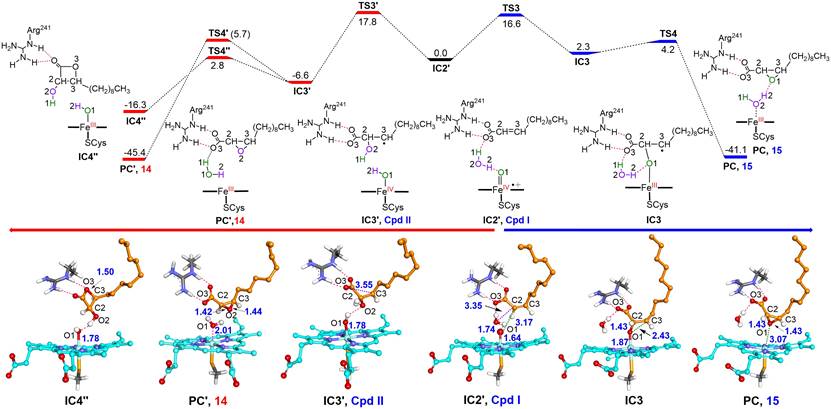
More surprisingly, in addition to the common epoxide products, undecanal was revealed to be the unexpected major carbon-carbon bond cleavage product of P450SPα and P450BSβ regardless the cis/trans-configuration of substrates. The combined isotopically labeled H218O2 tracing experiments, MD simulations and QM/MM calculations unraveled a highly uncommon mechanism for Compound I mediated aldehyde formation, which is initiated by the H-abstraction from the adjacent water derived from H2O2 activation. This mechanism is totally different from the C-C scission reaction of the saturated fatty acid by OleTJE, which is initiated by a typical substrate Cβ-H abstraction. Moreover,P450SPα and P450BSβ could also produce the corresponding fatty aldehydes from otherα,β-unsaturated fatty acids with different chain length.
The study not only proposes the key role of the active site water derived from H2O2 activation in the catalysis of CYP152 peroxygenases for the first time, but also expands the understanding of the mechanism of CYP152 peroxygenases for the C-C bond cleavage reactions. Of note, a high TON of 14,381 was achieved for P450SPα towards cis-2-dodecenoic acid with the in situ H2O2-generating system, providing a new, simple and more productive enzymatic system for aldehyde biosynthesis. Fatty aldehydes have long served as flavor and fragrance components, and also represent the essential metabolic intermediates for microbial synthesis of various industrially relevant oleochemicals, thus this work has broad prospects for practical application.
This work was supported by the National Natural Science Foundation of China, National Key Research and Development Program of China and the Natural Science Foundation of Shandong Province, China.
Links to this paper: https://onlinelibrary.wiley.com/doi/10.1002/anie.202111163
